The Chemistry of Chromium (Demo)
- Page ID
- 3058
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Chemical Concepts Demonstrated
- Cr2+, Cr 3+, and Cr (IV) oxidation states of chromium
Demonstration
|
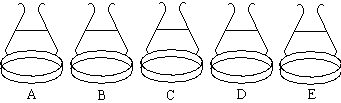 |
Observations and Explanations
| Dish | Observation / Explanation |
|---|---|
| A + B | Violet solution of Cr(H2O)6 3+. |
| C | The color of the solution changes from violet to an "acid green". |
| D | Green Cr(OH)3 precipitates from the green solution. More base will cause the solid to redissolve to give a green chromite Cr(OH)4 -solution. |
| E | The green solution changes to orange as the CrO42-/Cr2O72- ions are formed. |
| E + HCl | After the HCl is added a series of erratic color changes are observed. When the reaction is complete the solution is green. |
| A, C, & D with HCl | Solution A remains violet. Solution C changes from green back to violet. Solution D produces another green solution. |
| B with HCl & Zn | When the solution becomes acidic, several pieces of Zn are added to the dish. The bright blue color of Cr2+ (aq) will be visible momentarily, but air oxidation rapidly converts this to a green solution. |
| Original solution + BaCl2 + Pb(NO3)2 | A yellow precipitate of BaCrO4 will form. Adding HCl will redissolve the precipitate and produce an orange-yellow solution. Pb(NO3)2 produces another yellow precipitate, PbCrO4. |

